Work by Gases from different temperature gas inside flask
 |
| Pictorial demonstration. |
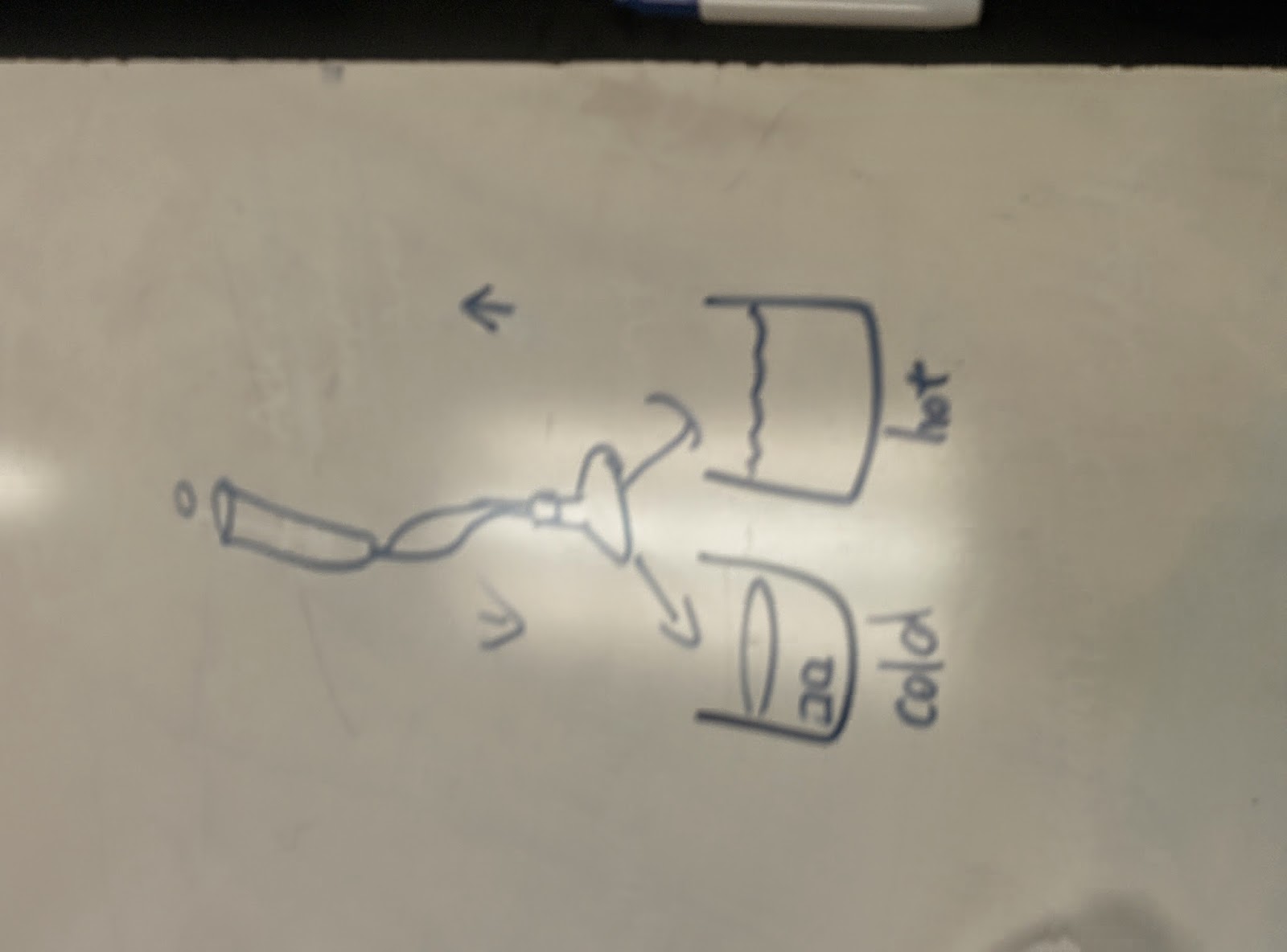 |
| The lab setup was to put flask to either hot or cold beakers of water. |
 |
| Setup Setup for work done by gas. as Flask full of air with increased temperature push up, or decreased temperature goes back down. |
Molecular Dynamic Applet presentation
*http://physics.weber.edu/schroeder/software/mdapplet.html
 |
| Dynamic Molecular Applet |
Fire Syringe
Note: The paper flashpoint of 451 degrees Fahrenheit is well known to readers of Ray Bradbury’s famous science fiction novel, Fahrenheit 451, about book burning for the sake of maintaining a stable society.
 |
| Fire Syringe lighting fire using by the adiabatic process. |
Radius .5 cm and volume calculation .49^2pi*initial or final air column
Final L= 1.97, wrong in the picture.
V1T1^5/2=VfTf^5/2
Tf=(H/Hf*T^5/2)^2/5=612
Compare to true value 451
percent error:(612 -451)/451= 31.5%
V1T1^5/2=VfTf^5/2
Tf=(H/Hf*T^5/2)^2/5=612
Compare to true value 451
percent error:(612 -451)/451= 31.5%


No comments:
Post a Comment